Ahead of the world theranostics day on March 31, I’m posting some ideas on how to advance theranostics with radiobiology.
We heard a lot of high profile acquisitions lately, such as Fusion by Astrazeneca for $2.4bn, RayzeBio by BMS for $4.1bn and Point Biopharma by Lilly for $1.4bn, so there is obviously a lot of interest in the field.
But first let’s get the basics out of the way so we have our definitions and understanding clear and everyone can follow.
The Beginning of Theranostics
Theranostics is a subfield within nuclear medicine that combines the words therapeutics and diagnostics to show that the same compound or drug can be used to both image the tumour (when combined with a gamma or positron emitter) and treat the tumour (when combined with a beta or alpha emitter).
The first very simple such compound is NaI (sodium iodide). I-123, which emits gamma radiation (photons) with energy 159 keV, is used for imaging with a gamma camera or SPECT/CT and it’s therapeutic pair is I-131, which emits beta particles (and photons), and is used for treatment of hyperthyroidism or thyroid cancer. A capsule of I131 is shown below.

Injecting radiopharmaceuticals into patients is nothing new. This first such treatment with I-131 was given in 1941, 83 years ago. Many more drugs have been developed since then to diagnose and treat various diseases, mainly cancer.
Theranostics is also referred to as theragnostics (notice the g from diagnostics) or radionuclide therapy or radiopharmaceutical therapy (RPT) or radioligand therapy or nuclear medicine therapy, but the idea is basically the same and has been for decades, and the various names are basically rebranding and marketing efforts.
Despite the long history of nuclear medicine, the moniker commonly given to nuclear medicine (unclear medicine), and the recent resurgence of theranostics, most other cancer treatment options (chemotherapy, hormone therapy, immunotherapy, surgery) cannot accurately quantify the benefit to risk ratio in each patient, prior to treatment. This feature is unique to External Beam Radiation Therapy (EBRT) and RPT, where the dose to tumour (benefit) and healthy tissue (risk) is accurately estimated (prior the treatment) and confirmed (after the treatment).
This quantification of dose to tumours and healthy tissue did not come about by luck, it required development of complex dosimetry software over years. Dosimetry software usage relies mainly on two completely different things: our understanding of radiobiology and our compliance with the law that requires optimization of therapy via dosimetry prior to each treatment. There is ample evidence that patient outcomes are improved when dosimetry is correctly implemented, and treatment is optimised for each patient, for both EBRT and RPT.
This is the personalized, optimised medicine everyone is talking about. But to quantify this risk to benefit ratio, first we need to talk about the different types of radiation in use and how they compare.
Types of radiation
Here’s a quick comparison of how different types of radiation can cause tissue or tumour damage, which is what radiobiology explains:
Type of radiation | Source example | penetration | Damage mechanism | Severity of damage |
Alpha particles | Ra-223 (xofigo) | Low (stopped by paper/skin) Max length in tissue 80μm | Ionisation, direct damage, | Very localised damage, high for close exposure (ingestion, inhalation, injection), low for external exposure. |
Beta particles | Y-90 (liver radioembolization), Lu-177-PSMA for prostate cancer | Medium (penetrate skin) Max length in tissue 1cm? | Ionisation, direct damage | More widespread damage than alpha, but less severe |
Gamma Rays & X-rays | Tc-99m, Diagnostic X-ray tube | high (penetrates entire body and is only partly attenuated) | Ionisation, indirect damage | Less damage than alpha/beta, more uniform and low damage that alpha/beta |
Mega Voltage | Linac used in radiotherapy | Very high (can penetrate thick lead walls) | Ionisation, indirect damage | Uniform medium damage in beam path |
Fast neutrons | Karmanos Cancer Center / Wayne State University | Extremely high (can penetrate lead, TVL in concrete 15cm) | Ionisation, indirect damage | Uniform high damage in beam path, same order of damage as alpha/beta |
Right now the field of RPT, which uses alpha, beta or gamma radiation, is still in the early stages, probably at the same stage EBRT was around 40 years ago when it was developing the technology around MegaVoltage therapy with clinical linear accelerators (linac). But RPT can and should learn from EBRT.
The benefits of RPT that uses alpha and beta particles for treatment is that damage is directly imparted to tissue in a smaller area compared to EBRT that uses MegaVoltage beams of photons or fast neutrons to indirectly cause damage in a wide area, via the creation of free radical and ionised molecules. See below how the ionisation looks along track length for alpha, X-ray and I-125.
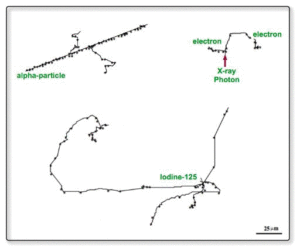
Why am I mentioning neutrons? In some ways, the high LET (Linear Energy Transfer) of neutrons is similar to that of alpha and beta particles.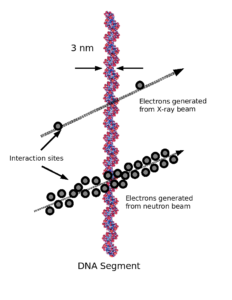
Around 10-20 years ago many expensive neutron cancer treatment centres popped up promising to be the next best thing. Now most of them have stopped or reduced patient treatments and a brief examination of the history may be useful for those who want to avoid repeating the same mistakes in RPT.
And one of the main reasons for the failure of neutron therapy is lack of understanding of radiobiology. Specifically it has been said that some of the lessons learnt were, and I quote:
- For any form of radiation therapy, radiobiological testing should be sufficiently comprehensive to identify strengths and weaknesses and include testing in many cell lines and tissues with different radiobiological characteristics and not just a few or one.
- In retrospect, better radiobiological modelling would have alerted clinicians of at least some of the adverse features and might have prevented the widespread use of fast neutrons, or at least restricted their use to specially defined situations.
Surprisingly some of those lessons apply to radionuclide therapy today, and to get the best treatment out of RPT that considers their penetration potential, damage mechanism, and severity of damage we need radiobiological models.
Dose-Effect Models
The concept of dose-effect relationships is crucial in radiobiology. It describes the connection between the amount of radiation exposure (dose) and the biological effect it produces. The most widely used model is the Linear No-Threshold (LNT) Model: This model proposes that any amount of ionising radiation, no matter how small, has the potential to cause some level of damage.
As British statistician George Box said, all models are wrong, but some are useful, and the actual dose-effect relationship can vary depending on factors like:
- Type of radiation: Different types of radiation deposit their energy differently, leading to varying degrees of damage.
- Dose rate: The rate at which radiation is delivered can influence the severity of effects.
- Cell type: Different cells have varying sensitivities to radiation damage. Some repair the damage more easily than others, some repopulate much faster than others
- Microenvironment and Oxygenation: What is surrounding the tumour? Is the irradiated tissue well oxygenated? If yes, then the damage from radiation is more effective. Tumours are known to be hypoxic (lack oxygen).
- Individual variability: Genetic factors and overall health can influence how individuals respond to radiation exposure.
The Linear Quadratic Model
The Linear-Quadratic model tries to take into account some of the above factors. Originally developed and applied for EBRT, dose is delivered in fractions (n) of around 2 Gy (d), α is the linear term related to single strand DNA breaks and β is the quadratic term related to double strand DNA breaks. Total dose D=n*d. The fraction of cells S surviving after such radiation is
![]()
To get a visual understanding of what the exponential means, see the graph below from 1968 comparing the surviving fraction of cells when exposed in vitro to various types of alpha particles, compared to 250 kVp X-ray.
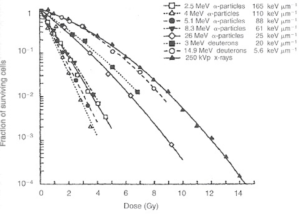
Based on EBRT, most healthy tissue has α/β < 4 Gy whereas tumours have α/β > 10 Gy so fractionation is beneficial because it spares healthy tissue while damaging tumours that have been irradiated with the same dose, as shown below. Yet despite the decades of treating cancer patients with EBRT, unknowns and uncertainties remain on the α/β and it’s applications. Some types of cancer have a very low α/β such as prostate cancer, mucosa cancer, melanoma, and liposarcoma so hypofractionation may be beneficial. RPT goes in exactly the opposite direction with hyperfractionation, and delivers the dose continuously in tiny dose rates compared to EBRT. Surprisingly, it has been shown to work well in prostate cancer.
The models simplicity should have alerted us that its predictions will not hold in some cases. Still its simplicity is what made it a mainstay since its introduction in 1966. Publications by QUANTEC (Quantitative Analysis of Normal Tissue Effects in the Clinic) and Emami reinforced the model as the best we currently have to understand radiobiology in EBRT, and various modifications were added to take into account repair and repopulation of cells. Understanding radiobiology and dose-effect relationships is crucial for establishing safe radiation exposure limits and developing effective cancer treatments for both EBRT and RPT.
Biologically Effective Dose
Absorbed Dose in Gy has been known for a long time that it does not correlate with patient response, but BED is correlated with patient response like normal tissue toxicity and tumour control, both in EBRT and RPT. The BED formalism from EBRT has been extended to RPT and there are various formulas that implement the α/β ratio from the LQ model, and other parameters like the repair rate μ to find BED in RPT for organs and tumours.
Looking at a specific example, it has been known from EBRT that the absorbed dose limit to the kidney is 23 Gy, whereas the BED is typically lower for a specific absorbed dose, and depends on radionuclide used, administration method and cycle number.
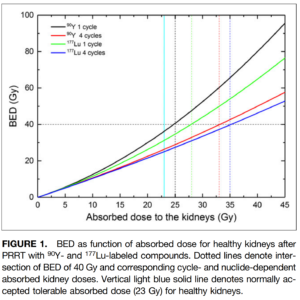
I know that accurate parameter values are hard to find, but again, the BED is the best metric we have right now.
Moving forward
Having established, widely recognised parameters to calculate BED in various situations is very important to advance the field of RPT forward.
Some have called for better reporting of dosimetry from clinical trials and plan to use that data along with adverse effects reported to establish different models, but to me that seems counterproductive and like putting the cart before the horse, and will explain why.
Despite the shortcomings of the LQ model, its parameters can be established reliably, easily, safely and practically, using various cell cultures in a lab. On the other hand, creating new complex models from clinical trial data will take years and is prone to many errors and confounding factors. Clinical trials are notorious for being expensive, require many patients, and their informed consent, and are mainly conducted with specific endpoints and objectives in mind, for example to approve a drug for RPT. Therefore clinical trials will only look at specific, limited radionuclides, doses, dose rates, cycles, organs and tumour of high interest during the limited time of a trial, and be confined by practical, financial and ethical limits. Many scenarios would not be studied due to limited resources within a clinical trial, whereas experiments with irradiated cell cultures are relatively quick and cheap.
The above does not mean that clinical trials do not have a place in testing the predictions of radiobiological models like the LQ model, but this testing in humans should be allowed to happen only after thorough testing in the lab. It is well known that lab results usually are not reproduced in clinical trials, and in the case of radiobiology, tumour characteristics. variability, heterogeneity, inhomogeneity and microenvironment play a big factor. Still cell cultures can help us make better decisions on what and how to test RPT in the clinic to improve efficacy. If we repeat the mistakes of neutron therapy, or copy the dose thresholds of organs from EBRT, we risk undertreating or delaying treatment to cancer patients with RPT.
The field of theranostics is relying on accurate, personalized dosimetry to deliver the best therapy, and for that we need radiobiological models based on cell cultures.
References
- https://iopscience.iop.org/article/10.1088/1361-6560/aaf26a/pdf
- https://jnm.snmjournals.org/content/jnumed/58/Supplement_2/97S.full.pdf
- https://www.rsna.org/news/2024/march/theranostics-for-cancer-patients
- https://pubs.rsna.org/doi/10.1148/rycan.220157
- https://pubs.rsna.org/doi/abs/10.1148/rg.230097?journalCode=radiographics
- https://jnm.snmjournals.org/content/65/3/362.long
- https://ascopubs.org/doi/10.1200/JCO.23.01241
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5669265/
- https://www.sciencedirect.com/science/article/pii/S1120179722014351


I agree that many radiobiological studies will be needed to advance theranostics.