The good
It all started in Israel in 1975 when Dr Levitt and Dr Warshel published their seminal paper “Computer simulations of protein folding”. Back then:
- computers were slow,
- Intel had released their 8-bit 8080 processor,
- you had to code in Fortran using punch cards,
- databases of molecules did not exist,
- nobody knew what a GPU was,
- Nvidia did not exist, and
- most chemists had to use a multiple flasks to get anything done.
In short, this kind of research was not easy. Some may say they used to walk to school uphill both ways.
Fast forward to now, and many things have changed:
- Dr Levitt, Dr Warshel and Dr Karplus won the Nobel Prize Chemistry in 2013
- high performance computers can distribute highly parallel workloads to multiple CPUs and GPUs, made mainly from the element Silicon, to perform in silico trials,
- Nvidia is a 2 trillion dollar company by market cap,
- virtual chemical libraries containing details of 10^10 molecules are constantly being updated via the internet,
- many abstract high level programming languages and packages exist that can extract features and patterns from big data to essentially teach the machine itself how to learn (machine learning),
- robotic automatic chemical synthesis and characterization can be found in labs and hospitals,
All of the above working together to advance computational chemistry and biology at light speed. Everything is now meant to be easy and fast.
With the power of those technological innovations, within the last 7 years or so many biotech start-ups were founded with one common vision: how to take the slow, expensive, and failure-prone process of drug discovery and make it cheaper, faster, more accurate.
There are indeed many things one would wish to change or even avoid in the complex way drugs are currently developed:
- testing in animals during preclinical trials
- testing in human subjects in phase I to IV clinical trials
- ethics committees
- regulatory oversight at every step of the way.
Most of the start-ups describe in silico or virtual trials as the process of using computer simulations, predictions and models to augment or substitute -in parts or whole- the drug discovery process, starting from the wet lab and finishing with phase IV clinical trials after the drug is approved, so new drugs can reach patients when they need them. Seems like a noble goal to me.
They all plan to revolutionize drug discovery, or so they say to their investors.
The bad
Unfortunately for everyone involved in virtual trials, as far as I know, no novel computer designed drugs have passed through the entire process yet. None are approved in clinical use by the authorities, in any country, yet. Perhaps it’s still too early. Perhaps we need to be patient, but both investors and patients are not and can not be.
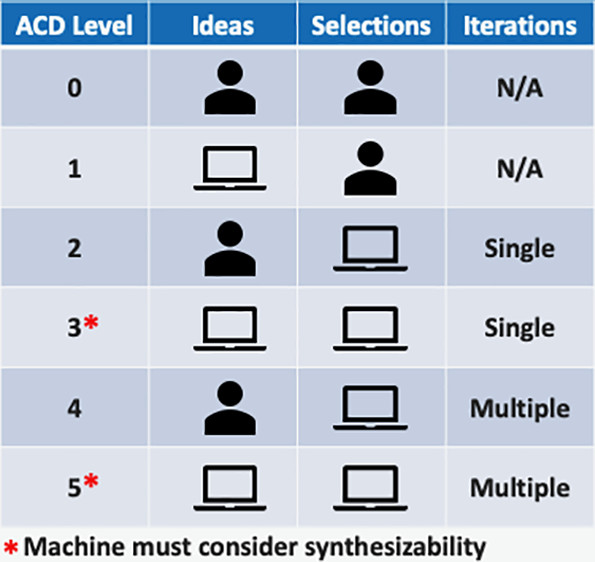
The definition for what constitutes a true success in computer automated chemical drug design may be unclear, but we are still far from a level 5 fully automated system as shown in figure 1 from Defining Levels of Automated Chemical Design. Until we get there, if we get there, hype and venture capital money will have to suffice!
At various points, we heard the hype or the good news, that computers found multiple new and promising drugs to treat Alzheimer’s or a new antibiotic. We heard that all the big tech players like Alphabet (Google) and Meta (Facebook) had created their own virtual trials labs. Those software (Alphafold and ESMfold respectively) competed in virtual drug competitions along with the small start-ups and university labs. Of course, big tech won, given the massive resources they can throw at anything. But the drug discovery process is more than just headlines and competitions.
We heard pharma is also very interested in the topic, without investing in this area more than other active research areas. Perhaps they are betting on all horses in the same race, not a winning strategy and that shows the lack of commitment by some parties involved.
That lack of commitment is justified though. What may have slipped under the radar is the general disappointment when few of the computer designed drug promises materialized. Some more realistic and down to earth headlines just did not make enough noise. For example, when Meta disbanded their ESMfold team to focus on money making products. It seems that most of the players in the drug design game (because for some this is a game, see FoldIt), broke the cardinal rule of keeping customers happy: you should under-promise and over-deliver, not over-promise and under-deliver.
Given the tight financial situation of start-ups in general, the high interest rates, the make or break moment may be sooner than expected. I think it’s time for the start-ups to show to the community (and most importantly their investors) some noteworthy results. Some do not want to disclose any details, for fear of intellectual property theft. But, as Pat Walters advocates in the video above, and his publications, a rising tide lifts all boats. After all, sharing information, code, data is the cornerstone of advancing science. (This turns out can be a bad thing in some cases, as we will see in the next section.)
Some start-ups say the problem is the size or quality of the molecular database, so they created bigger and better ones. Most say the problem is the accuracy of the modelling and ligand parameter prediction, so they claim they improved that (but how much is enough? how do you measure it?). Others say we still don’t have enough cheap processing power, so they think quantum computers are the solution (spoiler alert: they are not). Maybe this is similar to nuclear fusion, always a decade away. Always another problem to solve, and this time we will make it.
In my opinion, there are too many start-ups in this space and most of them will pivot to something related, or go bankrupt, but few may finally succeed in drug creation. I wish them all the best of luck, as long as they are not burning through my money until they make it!
The ugly
While many start-ups are throwing everything but the kitchen sink, to figure out how to safely, ethically, reliably, quickly, and accurately design drugs on a computer for the benefit of everyone, others have looked in the opposite direction.
Instead of making a drug as useful as possible with high benefit for patients and low toxicity, they used the same tools, databases, and models already developed, and looked for the most toxic molecules. With the click of a button a group created 1000s of chemical weapons, some known and some new, as proof of concept on how easily one can abuse the power of machine learning in drug discovery. They claim they did this to raise awareness of this issue, but what changed since the news broke out?
Creating something beneficial is orders of magnitude more complicated than creating something destructive, this is nothing new, all dual use technologies at first caused a lot of destruction before we learnt how to tame their power, regulate them and put them into good use.
Dual use
The combination of technologies described (databases, GPUs, machine learning etc) have undoubtedly many similarities with other well known dual use inventions that had been used for bad (at first) or for good (much later).
The first nuclear bomb was detonated in 1945, whereas the first nuclear power plant came online in 1954. Many more detonations followed some on testing sites and some on civilians, we all know the story, and consequences. Stockpiles of nuclear bombs continued to proliferate until effective methods to monitor nuclear explosions were implemented, regulators given enough power to monitor countries producing bombs, and the nuclear disarmament movement gathered support in 1986.
The GPS (Global Positioning System) was created by the US DOD in 1978 for navigating missiles. Most advanced long range missiles and munition produced since then have GPS guidance built in. We know why missiles are launched. Years later, in 1983, when the obvious applications of GPS in airplane safety were deemed necessary (following a deadly crash) was it open to civilian applications by president Ronald Reagan. Much later, the first company to offer GPS for car navigation was Mitsubishi in 1990 with Mazda’s Eunos Cosmo.
In short, it takes a lot of time, scientific effort, accurate risk assessment, political will, and public support for a technology to be used only for good. Are we there with virtual trials? What is still missing to make virtual trials something that can be used only for good?
That’s a difficult question. Taken into parts, there is nothing inherently wrong with a molecular database or a machine learning package or ordering materials from the internet to synthesize a compound. But together, and in the wrong hands, they can wreak havoc.
Pandora’s box is open
Some are now calling for controlling who can access the data and models mentioned, without hindering research and science. Seems like closing the stable door after the horse has bolted. Regulators can’t do much anymore.
The only hope now is that those with the knowledge to discover and synthesize weapons, understand this is unethical and unconscionable. However, those early pioneers had something completely different in mind. Is this an argument for an ethics course? Can morality be taught? When faced with technology like in silico virtual trials, how will we know that what we are developing does (or may do) more harm than good?
Until next time, take care!
References
- https://fortune.com/2024/02/19/sanofi-ceo-ai-promises-great-era-drug-discovery-fundamentally-change-medicine/
- https://www.nature.com/articles/d41586-023-02984-w
- https://www.ft.com/content/919c05d2-b894-4812-aa1a-dd2ab6de794a
- https://www.nature.com/articles/d43747-021-00045-7
- https://www.genengnews.com/topics/drug-discovery/drugs-dollars-and-data-recursion-eyes-cost-savings-from-ai-drug-discovery/
- https://www.engadget.com/ai-is-coming-for-big-pharma-150045224.html
- https://www.fiercebiotech.com/biotech/merck-finds-drug-discovery-dall-e-becoming-early-user-small-molecule-generative-ai-tool
- https://www.youtube.com/watch?v=HbfFS7bA5M0
- https://neurosciencenews.com/ai-personalized-medicine-25502/
- https://www.psu.edu/news/research/story/two-alzheimers-drugs-tested-head-head-first-ever-virtual-clinical-trial/
- https://www.fiercebiotech.com/cro/insilicotrials-ibsa-group-partner-expand-ai-use-drug-development-trials
- https://www.nature.com/articles/s42256-022-00465-9
- https://www.freethink.com/robots-ai/drug-discovery-alphafold
- https://www.sciencedirect.com/science/article/abs/pii/S1359644622004032